methyl acetate formation
Afterward the methoxide inter- mediate attacks a carbonyl group of the anhydride forming methyl acetate and the methylcarbonate ion Figure. Silica gel is the most widely used sorbent for the essential oils with solvents such as benzene or toluene chloroform methylene chloride ethyl acetate for development.
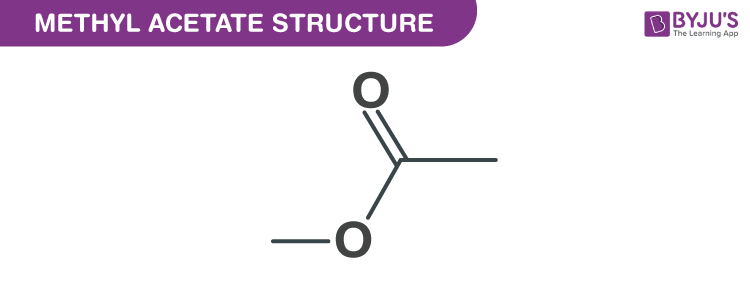
Methyl Acetate Structure Properties And Uses Of C3h6o2
Methyl acetate is produced industrially by liquid phase reaction of acetic acid and methanol in presence of an acid catalyst.

. Information on this page. When acetic acid and methanol it forms methyl acetate and water Ch3COOHCH3OH--CH3C2H3O2H20. Empirical formula Hills system for organic substances.
Methyl acetate can be further hydrogenated to ethanol which an important chemical and a fuel additive 1. Copy Sheet of paper on top of another sheet. Hydrolysis of Methyl Acetate.
Methyl acetate water mixture is produced in large quantities from purified terephthalic acid PTA plants. The acid catalyst used in this reaction was cone sulfuric acid. C 3 H 7 O 2 Molecular weight.
Acetic Anhydride by the Carbonylation Process. 923 Methyl acetate reaction data including standard Raman spectra of acetic acid methanol and methyl acetate. C 3 H 6 O 2.
100 1 rating Transcribed image text. At 57 C it is a colourless and flammable liquid which is used as a solvent for oils and many resins. When acetic acid and methanol it forms methyl acetate and water Ch3COOHCH3OH--CH3C2H3O2H20 What is methyl acetate used for.
Because of its toxicity however benzene can no longer be recommended as a solvent for TLC. Who are the experts. Experts are tested by Chegg as specialists in their subject area.
Methanol is burned with acetic acid in the presence of sulfuric acid to make methyl acetate. Used as a plasticizer. Copy Sheet of paper on top of another sheet.
Since methyl acetate is a comparatively low value solvent it has to be sold at a lower price. Methyl acetate can be made in a variety of ways some of them are listed below Carbonylation is one method that is utilized in industry. To produce methyl acetate methanol is heated alongside acetic acid in the presence of sulfuric acid.
The methyl acetate reaction takes place at 175C 350 F and 26 bars 380 psig pressure. Methyl chloroacetate C3H5ClO2 CID 7295 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more. Acetic acid methyl ester.
Use condensed structural formulas to write the equation for the formation of methyl acetate. C 3 H 6 O 2. Methyl acetate MA is produced by various methods such as the esterification of acetic acid the reaction between methanol and acetic anhydride the reaction between peroxycarboxylic acid and acetone and wood pyrolysis.
Use condensed structural formulas to. Methyl Acetate An Overview Sciencedirect Topics Thermodyn 1978 10 687-690. Used to manufacture Lubricants.
The manufacture of polyvinyl alcohol PVA also produces large quantities of methyl acetate 168 kg per kg PVA. Another method of production is the esterification of methanol and acetic acid in the presence of a strong acid. Methyl acetate is produced industrially via the carbonylation of methanol as a byproduct of the production of acetic acidmethyl acetate also arises by esterification of acetic acid with methanol in the presence of strong acids such as sulfuric acid this production process is famous because of eastman kodaks intensified process using a reactive.
The eluent tolueneethyl acetate 937 is suitable for the analysis and comparison of all of the important essential oils. We review their content and use your feedback to keep the quality high. Switch to calorie-based units.
What is the equation for formation of methyl acetate. Used for the biodegradation of organic materials. Used in the manufacturing of artificial leather.
Sulfuric acid is a common catalyst also used in this reaction. C 3 H 6 O 2 Structural formula as. The molecular or chemical formula of Methyl acetate is C 3 H 6 O 2.
C 3 H 6 O 2 Uses Methyl acetate Methyl acetate is used as a food additive to enhance the flavour of food. How is methyl acetate produced. Handling Storage Distribution Hazards Toxicity.
It is formed from the condensation of acetic acid and methanol. McMahon TB Formation Thermochemistry. Hence it would be a better.
It is a colourless volatile liquid with a pleasant odour and fleeting fruity like the taste. Carbon monoxide substrates are brought together in these reactions. Show transcribed image text Expert Answer.
Used in paint removers.
Synthesis Of Phenylmethoxycarbonylamino Methyl Acetate Via Ester Formation From Carboxylic Acid Chemsink

File Synthesis Of Methyl Acetate Svg Wikimedia Commons
Ethyl Acetate Molecule Of The Month March 2003 Html Version
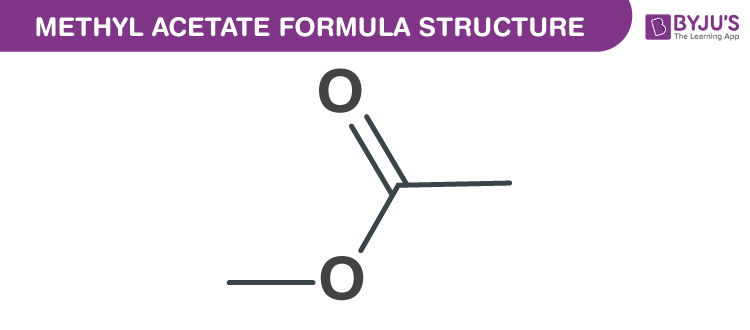
Methyl Acetate Formula Chemical Formula Structure Properties And Uses

Chemical Derivatization Of Acetate Using Methyl Chloroformate Mcf Download Scientific Diagram

Methyl Acetate An Overview Sciencedirect Topics

Methyl Acetate Metac 99 5 Solvents Wacker Chemie Ag

Methyl Acetate An Overview Sciencedirect Topics

Reactions Considered In The Formation Of Methyl Acetate By Alkylation Download Scientific Diagram

What Is The Balanced Equation For Methanol Ethanoic Acid Quora
What Smell Can I Get When Mixing Methanol With Acetic Ethanoic Acid Quora
Ethyl Acetate Molecule Of The Month March 2003 Html Version
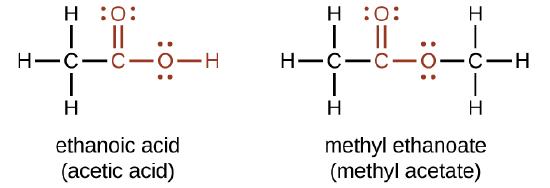
9 8 Carboxylic Acids And Esters Chemistry Libretexts

Mass Spectrum Of Methyl Ethanoate C3h6o2 Ch3cooch3 Fragmentation Pattern Of M Z M E Ions For Analysis
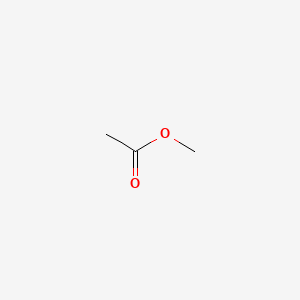
Methyl Acetate Ch3cooch3 Pubchem
What Is Reaction Of Methanol And Ethanoic Acid Quora

Reactions Considered In The Formation Of Methyl Acetate By Alkylation Download Scientific Diagram
File Synthesis Of Methyl Acetate Svg Wikimedia Commons

0 Response to "methyl acetate formation"
Post a Comment